From Anesthetic to Antidepressant: Ketamine's Surprising Journey
Part 1 of our series on ketamine’s role in mental health
For decades, the story of treating depression has been largely written by medications targeting the brain’s serotonin system. While these treatments have provided relief for millions, they represent only one chapter in a much larger and more complex book. For a significant portion of individuals—up to one-third of those with Major Depressive Disorder—standard therapies fail to produce adequate relief, leaving them in a state of treatment resistance. This frustrating reality has spurred a search for new approaches that go beyond conventional models.
Enter ketamine. A medication with a multifaceted history, ketamine is now emerging at the forefront of psychiatric innovation, offering a fundamentally different approach to healing the brain. This is Part 1 of our in-depth series exploring the clinical science of ketamine, from its unexpected origins to its profound impact on our understanding of mood and neurobiology.
A Surprising History: From the Battlefield to the Brain
To understand ketamine’s revolutionary role today, we must first look back to its creation. Developed in 1962 by chemist Calvin Stevens at Parke-Davis, ketamine was synthesized as a safer alternative to phencyclidine (PCP), an anesthetic known for causing severe, long-lasting psychotic reactions. Ketamine offered a powerful anesthetic effect but with a much shorter duration of dissociative effects and a superior safety profile.
Its remarkable ability to provide anesthesia without suppressing breathing or circulation made it invaluable. The FDA approved it for human use in 1970, and it quickly became a staple in emergency rooms and on battlefields, particularly during the Vietnam War, where it could be used safely in challenging and unpredictable environments. For years, ketamine’s primary identity was that of a surgical and emergency room workhorse.
The pivot into mental healthcare wasn’t planned; it began with a clinical observation. In the 1990s, researchers at the Yale School of Medicine began formally investigating anecdotal reports that patients with depression who received ketamine for anesthesia experienced a rapid, though temporary, improvement in their mood. This was no mere side effect; it was a profound clue. This pivotal research, particularly a foundational 2000 study published in Biological Psychiatry, demonstrated that a single, sub-anesthetic dose of ketamine produced robust and rapid antidepressant effects in patients with severe depression, sparking a revolution in psychiatric research.
The Brain on Ketamine: A New Pharmacological Paradigm
For over 50 years, the prevailing theory for depression focused on “monoamines”—neurotransmitters like serotonin, norepinephrine, and dopamine. But for the 30-40% of patients who do not respond to these medications, this model is clearly incomplete.
Ketamine doesn’t primarily affect serotonin. Instead, it targets the most abundant excitatory neurotransmitter in the brain: glutamate.
1. Beyond Serotonin: The Glutamate System
Glutamate is the master switch for brain signaling, responsible for over 90% of all excitatory connections in the human brain. It is the primary mediator of synaptic plasticity—the ability of synapses to strengthen or weaken over time—which underlies learning and memory. Chronic stress and depression are linked to a breakdown in this system, causing a loss of healthy neural connections (synaptic atrophy), particularly in regions like the prefrontal cortex and hippocampus. Ketamine intervenes directly in this pathway by acting as a non-competitive antagonist of the NMDA (N-methyl-D-aspartate) receptor, a key component in glutamate signaling.
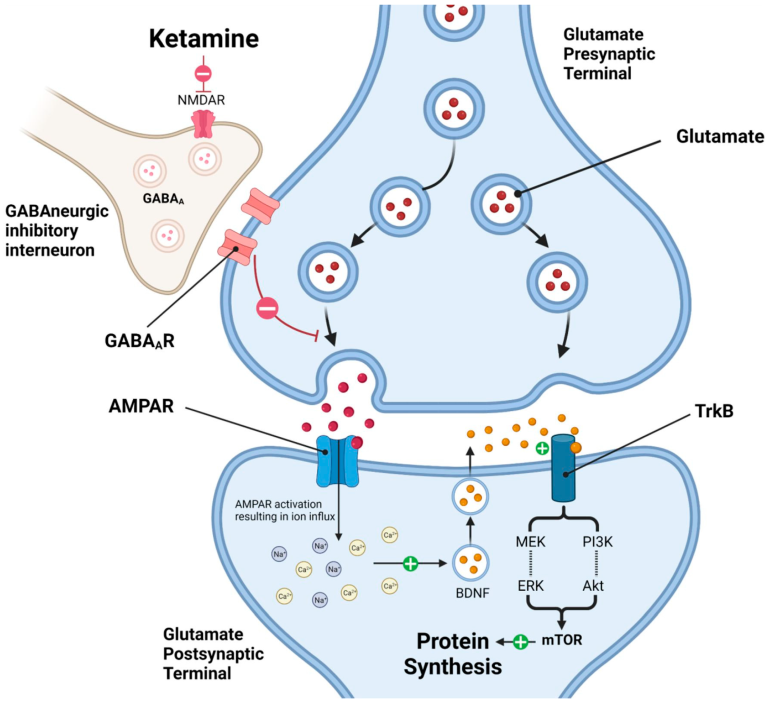
2. The “Brain Reset” and Synaptic Surge
Imagine a complex electrical grid. Chronic depression dampens this grid, making it sluggish and inefficient. Traditional antidepressants try to slowly increase the power supply. Ketamine, on the other hand, acts like a master reset switch. By temporarily blocking the NMDA receptor, particularly on inhibitory interneurons, it reduces their “braking” effect on the system. This leads to a surge of glutamate release.
This glutamate surge activates other, more responsive glutamate receptors—notably the AMPA receptor. The widespread activation of AMPA receptors is believed to be the critical trigger for ketamine’s antidepressant effects, initiating a powerful cascade that rapidly strengthens synaptic function and communication. It’s not a slow, gradual adjustment; it is a rapid, system-wide event that wakes up dormant circuits.
3. Promoting Neuroplasticity and Rebuilding Connections
This cascade of activity leads to the most profound aspect of ketamine’s action: it stimulates the production of Brain-Derived Neurotrophic Factor (BDNF).
BDNF is a powerful protein often described as “Miracle-Gro for the brain.” It is a key regulator of neuroplasticity, promoting the survival of existing neurons and encouraging the growth of new ones (neurogenesis) and, crucially, new synapses (synaptogenesis). Chronic stress is known to decrease BDNF levels, leading to atrophy in brain regions critical for mood regulation.
Ketamine’s ability to rapidly increase BDNF reverses this process. Within hours of administration, ketamine can trigger a measurable increase in synaptic proteins and the growth of new dendritic spines—the physical connections between neurons. This means ketamine doesn’t just alleviate symptoms by temporarily altering brain chemistry; it appears to activate the brain’s inherent ability to physically repair and rebuild the very circuits damaged by long-term depression.
This foundational science—from its anesthetic origins to its role as a master regulator of neuroplasticity—explains why ketamine represents such a monumental shift. It has provided researchers and clinicians with an entirely new framework for understanding and treating mood disorders on a timescale once thought impossible.
In our next post, we will take a closer look at ketamine’s most well-studied application and the condition that brought it into the spotlight: Treatment-Resistant Depression (TRD). We will explore the clinical data, the “gold standard” IV infusion protocol, and why it’s offering hope when other treatments have failed.
References:
- Rush, A. J., Trivedi, M. H., Wisniewski, S. R., et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry, 163(11), 1905–1917.
- Domino, E. F. (2010). Taming the ketamine tiger. Anesthesiology 113(3):p 678-684, September 1, 2010.
- Berman, R. M., Cappiello, A., Anand, A., et al. (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47(4), 351–354.
- Li, N., Lee, B., Liu, R. J., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964.


